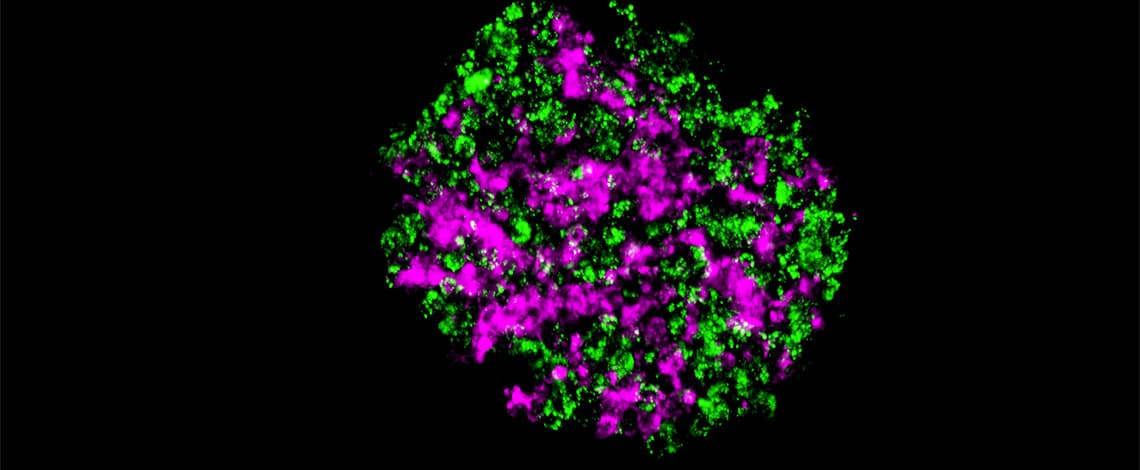
3D spheroid/organoid models are becoming the new standard for drug discovery applications because of their physiologically relevant characteristics relative to 2D cultured cells. However, in order that the spheroids/organoids function properly, it is necessary for the cells and the extracellular matrix (ECM) to be organized in the appropriate spatial arrangement to form microstructures, and how to create 3D spheroid models which mimic the environment in a human body is a hot topic. Nobuhiko Kojima and his group at Yokohama City University, Japan, are developing the technologies of designing different types of spheroids with internal microstructures.